
- #PERIODIC TABLE CHEMISTRY ELECTRON CONFIGURATION FULL#
- #PERIODIC TABLE CHEMISTRY ELECTRON CONFIGURATION PLUS#
Each element is uniquely defined by its atomic number.Ītomic mass: The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Atomic number: The number of protons in an atom.
#PERIODIC TABLE CHEMISTRY ELECTRON CONFIGURATION FULL#
The table below shows the full forms of the electron configurations of noble gases. For an explanation of these aspects, see the reference by Schwarz listed below. Thus, the electron configuration of Sc is 3d 1 4s 2. Starting with Scandium (Sc, atomic #21), the 3d orbital has a lower energy than the 4s. Energy levels and sublevels Principal energy level Therefore, orbital 4s is filled with electrons prior to orbital 3d. The reason for this is that the energy level of orbital 4s is slightly lower than that of orbital 3d. Note that in the electron configuration of both K and Ca, the 4s orbital is filled before the 3d orbital. The one additional electron configuration completes the picture for 19 electrons of Potassium.
#PERIODIC TABLE CHEMISTRY ELECTRON CONFIGURATION PLUS#
The abbreviated form - 4s 1 - means the electron configuration of Argon (Ar), plus one electron in the 4s orbital. The full electron configuration of Potassium (K) is 1s 22s 22p 63s 23p 64s 1. Thus, the configuration shown for Potassium is 4s 1 (see Table below). Thus, substituting the config of He gives the full config for Neon: 1s 22s 22p 6įor example, for Potassium (K) (atomic #19), the preceding noble gas is Argon (Ar) (atomic #18). For example, the abbreviated configuration for Neon is 2s 2 2p 6. In the periodic table beyond Helium (He), each element's electron configuration is shown in an abbreviated form that starts with the symbol of the noble gas that precedes it. The superscript shows that there is one electron in the 1s orbital. The simplest configuration is for Hydrogen: 1s 1. Notice that both of these ions now have 18 electrons just like the Noble Gas, Argon.Įlements with the same # electrons or electron configurations are said to be isoelectronic.An atom's electron configuration describes the distribution of its electrons in the atomic orbitals ordered by the orbitals' energy levels.
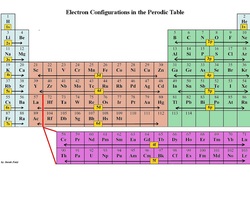
Lastly, the exponent, or number of electrons spins, the magnetic spin quantum number, m s, will indicate how many blocks across that section to count in order to reach the destination electron. The second value, the angular momentum quantum number, l, indicates the section of the periodic table where the electron is located (s, p, d, f).

The first value is the principle quantum number, n, which indicates the row on the periodic table. Imagine finding your seat without your ticket!Įlectrons are distributed throughout sections of atom much like seats in a stadium and the electron configuration acts as the ticket, giving directions to find a specific electron’s atomic address using the periodic table as the seating chart. Just as a stadium has seating chart, separating various sections, so does an atom.Ī ticket will help identify the section, row, and seat that of each spectator, specifically. Think of it as an address for each electron within an atom.


 0 kommentar(er)
0 kommentar(er)
